Lonza Group Ltd., a Swiss pharmaceutical contract manufacturing organization, is joining forces with Oxford Nanopore Technologies plc., a pioneering company in nanopore sequencing, to enhance the analysis of mRNA therapeutics. This collaboration aims to create and commercialize a groundbreaking test designed to evaluate various essential quality characteristics of mRNA products more efficiently.
mRNA technology has gained significant attention lately, especially for its crucial role in the swift development and distribution of COVID-19 vaccines. However, the process of analyzing mRNA products, including the indirect sequencing of mRNA, has been notably slow and labor-intensive. Recognizing the need for improvement, Lonza and Oxford Nanopore have decided to work together to advance the field of mRNA therapeutics, which are key in fighting infectious diseases, cancer, and genetic abnormalities. Their joint effort will focus on the cGMP (current Good Manufacturing Practice) validation and market launch of an innovative test that directly sequences both the DNA template and the mRNA molecule itself.
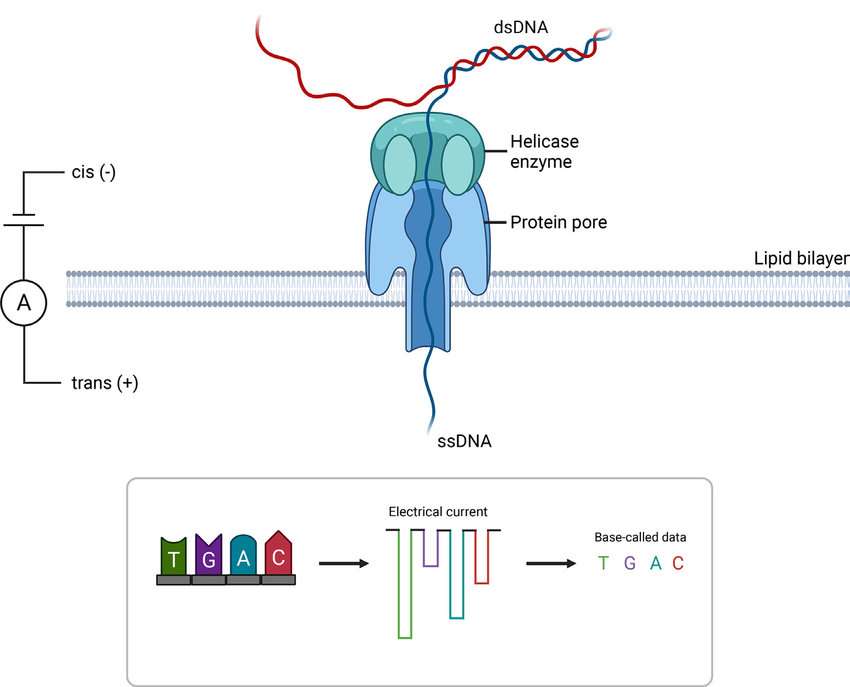
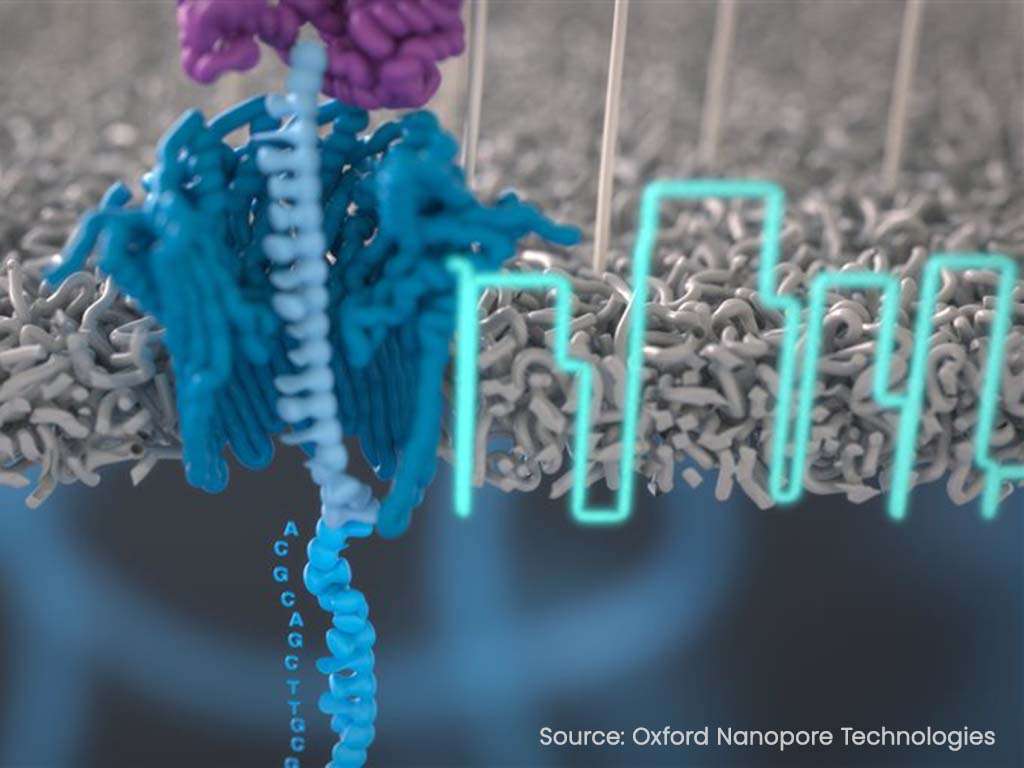
Utilizing Oxford Nanopore’s technology, capable of directly sequencing native RNA molecules, the partnership aims to streamline the quality control processes in mRNA production. The technology allows for the measurement of various mRNA quality attributes simultaneously at the production site using a single platform, potentially reducing the time required for analytical testing and offering a strategic advantage in the production of mRNA therapies. Torsten Schmidt, who leads Lonza’s mRNA business unit, highlighted the growing demand for mRNA products and the limitations of existing analytical methods. He noted that improved analytics could simplify regulatory processes and expedite the development of new therapies.
As part of this collaboration, Oxford Nanopore will share its in-house developed workflows with Lonza for GMP validation. After this step, Lonza plans to integrate the sequencing technology into its suite of analytical development services, thereby amplifying the impact of this partnership in the realm of mRNA technology. This initiative is poised to play a critical role not only in response to future pandemics but also in the continuous advancement of mRNA therapeutic research and development.
Source: European Biotechnology, Oxford Nanopore Technologies
No related posts.