The market for lithium-ion batteries manufacturing has shown persistent high-growth rates over the previous decades, thanks to the rise of electromobility and the subsequent expansion in EV manufacturing. As Europe and the rest of the world strive towards zero-emission targets, the global lithium-ion manufacturing capacity is predicted to see unprecedented demand for battery raw materials and manufacturing capacities in the coming decade. Simultaneously, battery research and quality control needs must keep pace with the increased demand for energy storage capacity.
In my recent blog post Lithium-ion Battery Manufacturing and Quality Control – Part 1, I discussed the economic landscape in the lithium-ion battery market, growth forecast and analytical requirements in quality control and monitoring, as well as technologies involved in battery testing and material analysis. In this post I will take a deep dive into some applications and technologies that support quality control and testing of battery material.
Outline
ToggleAnalytical requirements in manufacturing process, quality control and monitoring
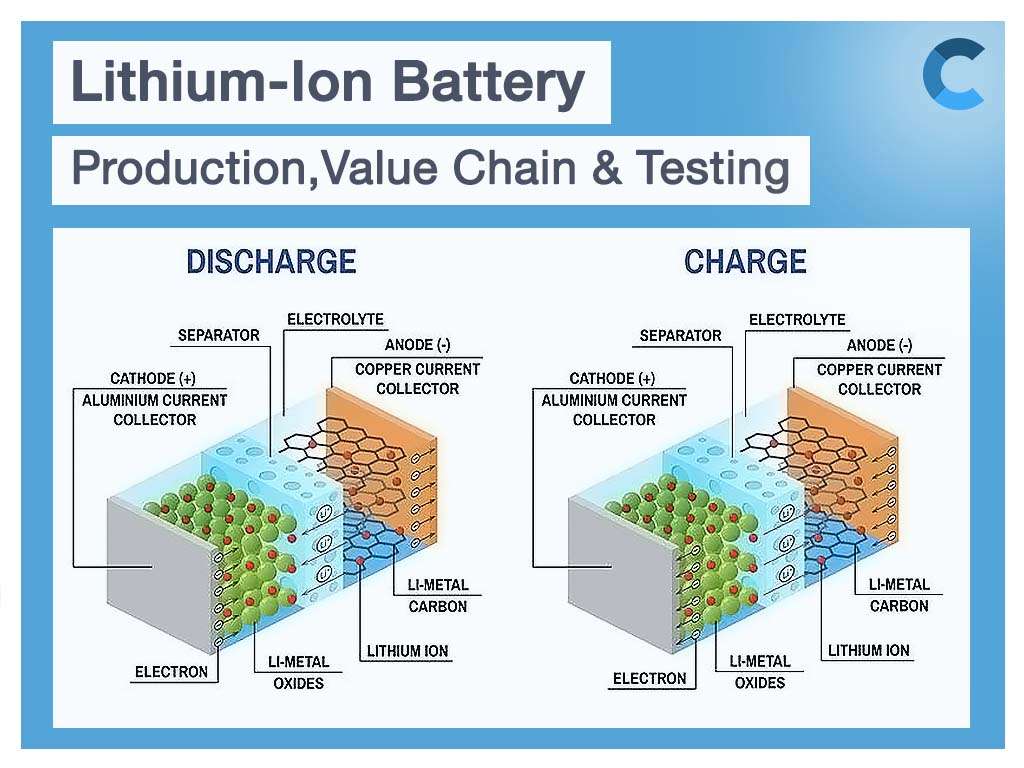
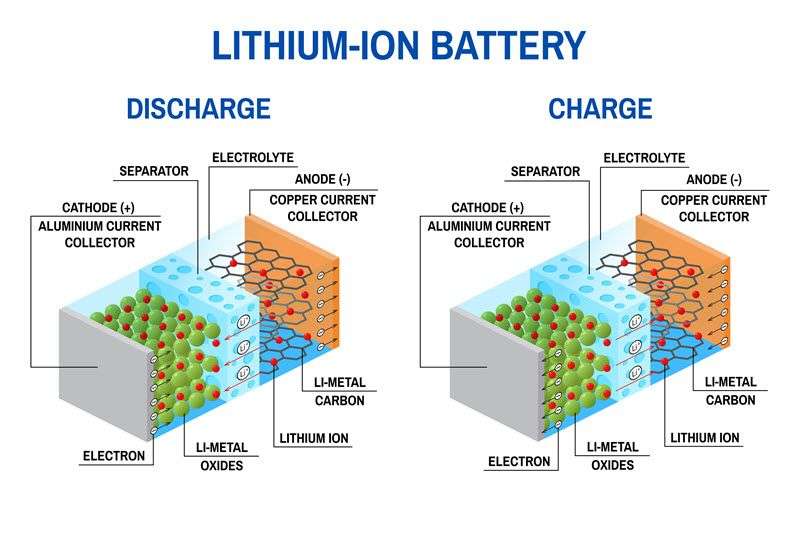
As mentioned in my previous blog post, there are three main components of a battery: two terminals made of different chemicals (typically metals), the anode and the cathode, and the electrolyte, which separates these terminals. The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. The infographic below provides an overview of the battery structure, including Li-ions and electrons flow during charge and discharge.
During manufacturing, battery producers must not only deliver consistent overall quality, but they must also deliver it throughout the manufacturing process. Likewise, development of new battery materials must ascertain all the critical parameters that could affect battery performance throughout the entire manufacturing process. Let’s evaluate the main components of a battery, cathode, anode, and electrolyte and investigate why quality is important to ensure performance.
Battery cathode material and elemental composition
A cathode’s active material is composed of lithium and, in the majority of the cases, one or several metals. Active materials have different characteristics depending on the type and proportion of metal in the cathode. For example, Ni (nickel) has high capacity, Mn (manganese) and Co (cobalt) have high safety, and Al (aluminum) increases the power of a battery. A cathode typically consists of a lithium transition metal oxide such as lithium-cobalt oxide (LiCoO2, LCO) and lithium-manganese oxide (LiMnO2, LMO) coated on metal foil (copper or aluminum).
The preferred cathode materials for EV batteries, on the other hand, are nickel-cobalt-aluminum oxide (Li(NiCoAl)O2, NCA), nickel-manganese-cobalt oxide (Li(NiMnCo)O2, NMC), and lithium-iron-phosphate (LiFePO4, LFP). It is fair to say, however, that battery development is a dynamic market and other types of batteries with a distinct cathode material structure are at the forefront, as for instance the Na-ion battery that was introduced by CATL in 2021.
Battery manufacturing process and battery testing
Typically, mixtures of these materials are applied commercially with varying contents. The proportion and content of the main elements in the cathode material can affect the performance and cost of the lithium battery significantly, and the content of impurities in the material alters the safety of the battery. Purity of the cathode material is critical, and changes in the raw material processing or synthesis can cause the introduction of impurities in the final cathode material. Conclusively, accurate determination and quantification of the main elements, as well as trace impurities, becomes particularly important.
Here, the inductively coupled plasma optical emission spectrometry (ICP-OES) provides a rapid detection method for the determination of major elements and trace impurities in material used in lithium batteries. The application notes below demonstrate a fast analytical method for the determination of major and trace elements in the ternary cathode material of lithium batteries using the Thermo Scientific™ iCAP PRO Series ICP-OES. The notes describe the method development as well as presenting key figures of merit, such as detection limits and stability.
Application note: Sensitive determination of elements in lithium batteries using the ICP-OES
Application note: Determination of elemental impurities in lithium iron phosphate using ICP-OES
Battery anode material and elemental impurities
A state-of-the-art lithium-ion battery anode is commonly graphite-based. Though other carbon-based materials, such as graphene or silicon-based materials, tin-based materials, and metal oxides have been developed and may provide an alternative for next-generation batteries. Currently, most anode material is generally made from graphite powder.
Graphite powder is suitable for this application primarily because it is an easily molded, chemically stable, and non-metallic material with good electrical conductivity and high temperature, oxidation, and corrosion resistance. It also has a large lithium-ion diffusion coefficient with a high lithium insertion capacity and does not change volume with insertion of lithium ions. In addition, graphite powder can be modified through various oxidation and pyrolysis processes to generate a core-shell structure that can improve its charging/discharging performance and increase the anode lifetime. Therefore, graphite powder has become the main lithium-ion battery anode material in use today in smaller consumer goods, such as mobile phones, as well as in electric vehicles.
To ensure the quality of graphite as anode material, ICP-OES can provide a robust, accurate and reliable analytical method for the determination of various trace elements in graphite material for lithium-ion batteries. Application of ICP-OES provides the advantages of high sensitivity, good stability, fast analysis speed and low operating expenses required for all aspects of elemental analysis in the lithium-ion battery workflow. The application note below demonstrates the performance of the Thermo Scientific™ iCAP™ PRO X ICP-OES Duo instrument for quantitative trace element impurity analysis in graphite powder samples used for lithium-ion battery anode production.
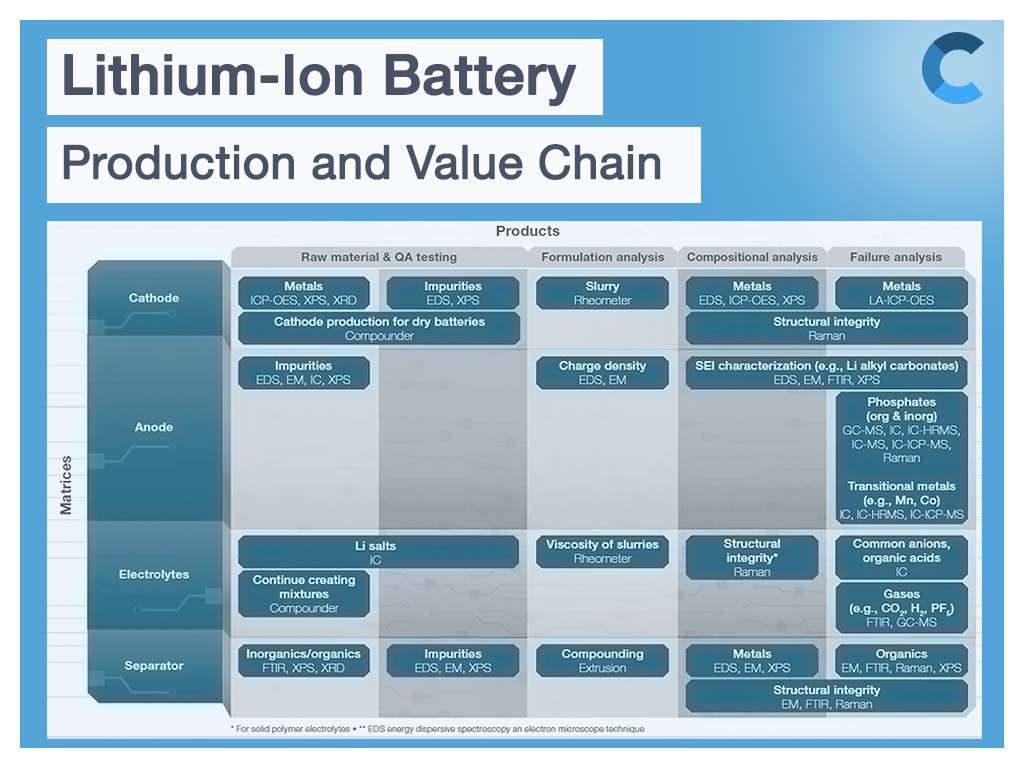
Electrolyte impurity analysis during battery production
Electrolyte plays a key role in transporting the positive lithium ions between the cathode and anode, and consequently the charging and discharging performance of the battery. Hence, it needs to be checked for potential impurities. At the same time, the electrolyte is also a sample type that allows the investigation of aging processes, as degradation products from all components of the battery can accumulate within it over time.
The electrolyte consists of a conducting salt in an organic solvent. The most common electrolyte salt is lithium hexafluorophosphate (LiPF6), but there are also lithium perchlorate (LiClO₄), lithium tetrafluoroborate (LiBF₄), lithium hexafluoroarsenate (LiAsF₆), lithium hexafluorosilicate (LiSiF₆), and lithium tetraphenylborate (LiB(C₆H₅)₄). The electrolyte in lithium-ion batteries is often a mixture of lithium salts and additional organic solvents. Some organic solvents used in the electrolyte solution are ethylene carbonate, diethyl carbonate, dimethyl carbonate, ethyl methyl carbonate, propylene carbonate, methyl formate, methyl acrylate, methyl butylate and ethyl acetate. Additives such as vinylene carbonate (VC) and fluoroethylene carbonate (FEC) are commonly added to the electrolyte to improve the overall performance of lithium-ion batteries.
Considering the chemical compound variety in the electrolyte (salts, ionic species, organic solvents, metals, etc.), different analytical techniques are required depending on analytical challenge. For instance, anions of lithium salts can be determined by ion chromatography (IC) to ensure that the solutions have been prepared at the proper concentrations. Gas chromatography or gas chromatography–mass spectrometry (GC-MS) can be used to provide the qualitative and quantitative composition of organic solvents in the electrolyte and aging byproducts. The inductively coupled plasma optical emission spectrometry (ICP-OES) provides robust and sensitive measurement of trace element impurities. Investigate the application notes below for a better understanding of how those techniques can be used for electrolyte analysis and quality testing.
Application note: Robust and sensitive measurement of trace element impurities in LiPF6 electrolyte…
Case study: Orbitrap GC-MS Technology Provides New Insight into Lithium Ion Battery Degradation
As you can see, many analytical methods can be applied for quality testing of battery material. If you’d like a better overview of the available tools and techniques for chemical, elemental, and structural analysis, please visit our “Advanced battery technology enabled with Thermo Scientific tools and instruments” page or discover additional resources below.
Original article: https://www.thermofisher.com/blog/analyteguru/challenges-in-lithium-ion-battery-manufacturing-and-quality/
Frequently asked question
How has the battery manufacturing process evolved with the rise of electric vehicles?
The surge in electric vehicles (EVs) has significantly influenced the battery manufacturing process. With EVs demanding higher energy density, longer lifespan, and rapid charging capabilities, the battery industry has had to adapt. This includes refining the electrode manufacturing process, optimizing the mixing and coating processes, and ensuring the electrolyte filling is precise.
The development of battery systems tailored for EVs has led to innovations in cell design and manufacturing methods. For instance, the production of lithium-ion cells suitable for EVs often requires a different manufacturing process than those for smaller electronics. Key processes, such as the calendaring process and cell formation, are meticulously controlled to ensure optimal cell performance. As the battery manufacturer’s role becomes increasingly crucial in the automotive sector, continuous advancements in manufacturing concepts and production equipment are expected.
What are the different types of battery cells, and how does their manufacturing differ?
There are various types of battery cells, including cylindrical cells, pouch cells, and prismatic cells. Each type has its unique manufacturing process, from electrode coating to cell assembly. For instance, cylindrical cells involve winding the coated electrodes, while pouch cells require a flat stacking method.
Depending on the cell format and design, the manufacturing plant might employ different manufacturing processes and equipment. The choice of cell size, cell case, and even the welding process for cell tabs can vary. Moreover, the energy density of the battery, a critical factor for applications like EVs, might influence the choice of active materials and the electrode manufacturing process. As the battery industry evolves, we’re also seeing the emergence of new battery types, such as solid-state batteries, which come with their unique manufacturing challenges.
How do manufacturing methods impact the energy density of the battery?
The energy density of a battery, which determines how much energy it can store for a given volume, is significantly influenced by the manufacturing process. Factors like the quality of the electrode coating process, the precision in electrolyte filling, and the effectiveness of the calendaring process play a crucial role. The choice of active material, such as specific metal oxides for the cathode, also impacts energy density.
Different manufacturing processes can yield varying energy densities, even with similar materials. For instance, the electrode manufacturing process, especially the coating and drying processes, needs to be optimized to ensure uniformity and minimal defects. Process parameters, such as the drying temperature and the speed of the production line, can influence the final energy density of the battery. As the demand for higher energy density grows, especially in sectors like EVs, refining manufacturing processes becomes paramount.
What challenges do battery manufacturers face in scaling up production capacity?
Scaling up battery production capacity presents several challenges. These include ensuring consistency in the manufacturing process, managing supply chains for raw materials like lithium and other metals, and maintaining quality control across larger production volumes. Additionally, the integration of new production equipment and technologies, while maintaining process efficiency, can be complex.
As battery manufacturers aim to meet the rising demand, especially from the EV sector, they must consider various factors. The entire manufacturing process, from electrode production to cell finishing, needs to be scalable without compromising cell performance. Manufacturing contributes about 25% of the battery’s cost, so optimizing manufacturing processes could lead to significant cost savings. Moreover, as new battery technologies emerge, manufacturers must be agile in adapting their production lines and manufacturing concepts.
How do manufacturers ensure safety and quality in the battery manufacturing process?
Safety and quality are paramount in the battery manufacturing process. Manufacturers employ rigorous testing methods, from checking individual cell performance to testing entire battery packs. Processes like cell formation and battery testing ensure each unit meets the desired specifications. Additionally, the integrity of seals, welds, and other components is regularly inspected to prevent failures.
The battery manufacturing process involves several steps where quality can be compromised, from the mixing process of electrode materials to the final cell assembly. Depending on the cell design and type, different manufacturing processes and testing equipment are employed. For instance, pouch cells require thorough inspection to ensure the pouch’s seal integrity. Continuous process monitoring, combined with periodic audits and inspections, ensures that the batteries produced are both safe and reliable.
Additional resources
Webinar: Powering Up Battery Materials Analysis using ICP-OES and ICP-MS
Website: Elemental Analysis Solutions for Battery Material Testing
Website: Battery technology research enhanced with electron microscopy and spectroscopy
Blog Post: Challenges in Lithium-ion Battery Manufacturing and Quality Analysis – Part 1
Blog Post: Is Battery Technology Just All About Lithium?
Blog Post: Making Energy Greener – What Role Does Elemental Analysis Play? Part 1
Join the conversation: Discover how to perform elemental and structural analysis in battery material testing
No related posts.