The automotive industry is about to undergo a significant transformation thanks to the electric vehicle (EV) revolution, which is being pushed by the need to decarbonize personal transportation to fulfill global targets for greenhouse gas emission reduction. Consequently, demand for lithium-ion batteries, battery cells and battery packs for EVs is rising rapidly.
Global installed battery storage capacity and battery cell manufacturing is forecast to expand by 30-40% annually in the next five years. A trend that is likely to continue. By 2030, it is expected to reach 9,300 gigawatt hours, which is a 20-fold increase from 2020 levels.
Outline
ToggleLithium-ion battery production capacity
With the rise of electromobility and the consequent increase in EV manufacturing, the market for lithium-ion batteries and cell production has seen consistently high-growth rates. For that reason, developing domestic battery supply chains, including battery manufacturing capacity, is becoming increasingly important as countries strive to shift away from internal combustion vehicles to electric mobility.
China is by far the leader in the battery race in 2023 with about 60% of global battery manufacturing capacity. It is followed by the United States with only 6%, or 44 GWh (Source: S&P Global Market Intelligence). European countries collectively make up for 68 GWh, or around 10% of global battery manufacturing.
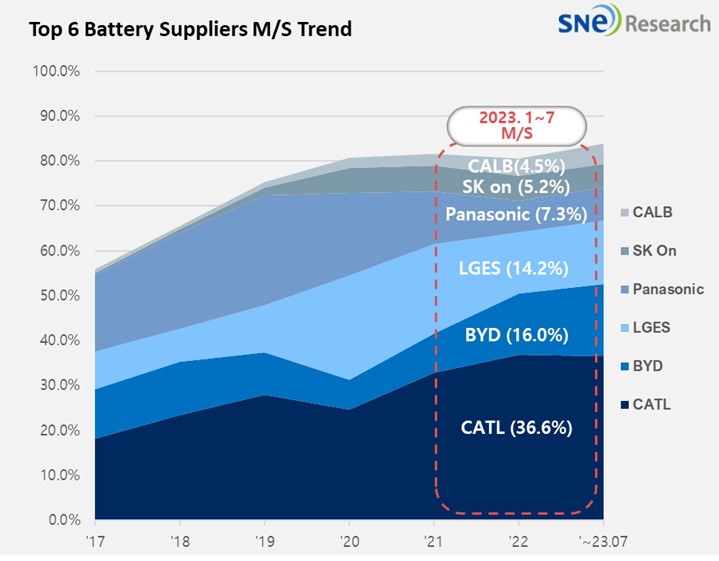
Source: SnereSearch
Global lithium-ion manufacturing capacity is predicted to more than double by 2025, propelling the industry toward zero-emission targets. China is likely to come out on top, with an estimated global capacity of roughly 65%. European countries are rapidly scaling up battery cell production. For example, Germany’s capacity is expected to increase by 15 times in four years, reaching 164 GWh.
It is crucial to note that the battery sector is rapidly evolving, and current projections may alter owing to economic or political factors. However, both battery demand and manufacturing capacity are expected to rise.

Table: Top 10 countries by estimated lithium-ion battery manufacturing capacities in 2025 (Source: S&P Global Market Intelligence).
Analytical requirements in quality control and battery testing
There are three main components of a battery: two terminals made of different chemicals (typically metals), the anode and the cathode, and the electrolyte, which separates these terminals.
The electrolyte is a chemical medium that allows the flow of electrical charge between the cathode and anode. Increases in battery performance require the development of new battery components, as well as understanding and addressing the mechanisms that result in performance degradation with repeated charging and discharging cycles.
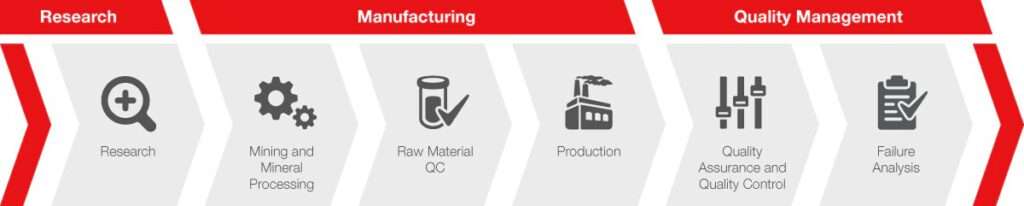
Battery manufacturers must not only deliver consistent overall quality, but they must also deliver it throughout the manufacturing process. Quality needs to be checked at every stage of production, from raw materials to cell assembly, to keep production efficient and reduce waste. Likewise, development of new battery materials must ascertain all the critical parameters that could affect battery performance throughout the entire manufacturing process.
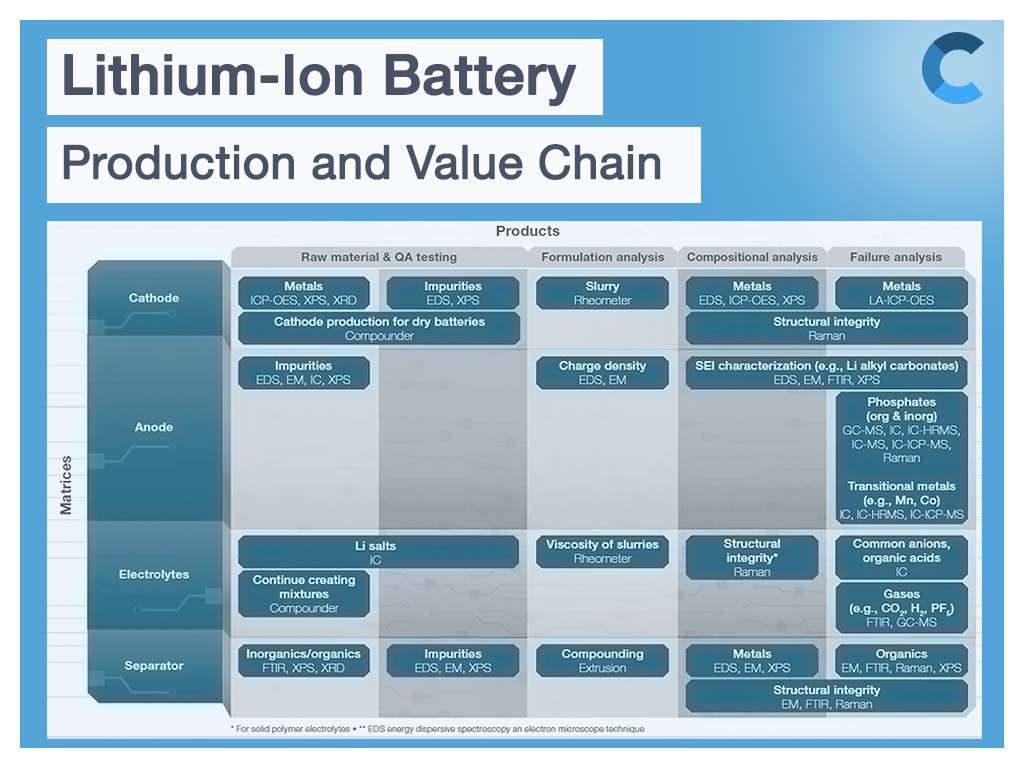
The infographic below provides a great overview of the solutions for physical, chemical and structural analysis of cathode material, anode material, electrolyte, and separator material and structure.

Overview of the solutions for physical, chemical and structural analysis of cathode, anode, electrolyte, and separator material and structure.
Benefits of each analytical technique
Evaluation of batteries and battery components requires various analytical methods that study materials and component surfaces at various scales. In this section, I would like to briefly highlight the benefits of various analytical techniques, including:
- mass spectrometry, as ICP-OES & ICP-MS, GC-MS, IC-MS
- X-ray photoelectron spectroscopy (XPS)
- electron microscopy (SEM & TEM)
- molecular spectroscopy, as FTIR, Raman and NIR
- micro-computed tomography (microCT)
- nuclear magnetic resonance (NMR)
- X-ray diffraction, X-ray fluorescence
- rheometry, viscometry, and extrusion.
Elemental composition and impurity analysis in battery production process
Deviations in chemical composition or impurities in electrode materials can significantly impact final battery performance. For this reason, chemical composition and elemental impurity analysis are an integral part of the battery manufacturing process.
The often-used inductively coupled plasma (ICP) as optical emission spectrometry (ICP-OES) or mass spectrometry (ICP-MS) is one of the most accurate and reliable tools to measure elemental composition and impurity analysis of cathode and anode material. The instrumentation of ICP is used to measure impurities in the electrolyte to a ppt level. Another way to analyze elemental composition and detect impurities is through X-ray fluorescence (XRF) solutions.

Structural analysis of lithium battery components and cell assembly
By combining analytical techniques such as micro-computed tomography (microCT), scanning and transmission electron microscopy (SEM and TEM), DualBeam (focused ion beam SEM; FIB-SEM), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and advanced 3D visualization and analysis software, researchers can obtain the critical structural and chemical information they need to build better batteries.
With this multimodal information at multiple length scales, researchers can learn fundamental properties of the battery as it changes throughout its lifetime, leading to major breakthroughs in battery design. These details could range from how different components fail as the battery is used to how lithium migrates between electrodes. Find out more about our Battery technology research enhanced with electron microscopy and spectroscopy.
Lithium-ion battery recycling – an emerging field of concern
Growing numbers of electric vehicles and stationary storage capacities present a serious waste-management challenge for recyclers at end-of-life. This waste presents numerous serious challenges of scale. Nevertheless, spent batteries may also present an opportunity to recover strategic elements and critical materials for key components in electric-vehicle manufacture. Elements and materials contained in electric-vehicle batteries are not available in many nations.
Access to resources is crucial in ensuring a stable supply chain. Recycled lithium-ion batteries from electric vehicles will therefore provide a valuable secondary source of materials. The recycling process is far from simple, however. This Nature publication, “Recycling lithium-ion batteries from electric vehicles,” nicely highlights challenges in lithium-ion battery recycling.
Continue to Lithium-ion Battery Manufacturing Process and Quality Control: Part 2 >
Original blog post: https://www.thermofisher.com/blog/ask-a-scientist/challenges-in-lithium-ion-battery-manufacturing-and-quality-analysis-part-1/
Additional resources
Challenges in Lithium-ion Battery Manufacturing and Quality Analysis – Part 2
Elemental Analysis Solutions for Battery Material Testing
Battery technology research enhanced with electron microscopy and spectroscopy
Advanced battery technology enabled with Thermo Scientific tools and instruments
Frequently asked questions
What is the significance of lithium in battery cell manufacturing?
Lithium, a lightweight metal, plays a pivotal role in the manufacturing process of battery cells. It serves as the primary active material in the cathode of a lithium-ion battery cell. The unique properties of lithium, such as its high-energy density and ability to undergo repeated charging and discharging cycles, make it an ideal choice for battery production. The electrolyte in these batteries often contains lithium ions, which move between the anode and cathode during the charging and discharging processes.
In the realm of lithium-ion batteries, there are various cell designs, such as cylindrical cell and pouch cells. Depending on the cell design and specific application, the amount, and type of lithium metal or lithium metal oxide used can vary. The production of lithium-ion batteries involves several key processes, including the mixing process of electrode materials, coating and drying, and the formation process. All these steps ensure the electrochemical performance of the battery is optimized.
How does the electrode manufacturing process impact battery cell performance?
The electrode manufacturing process is a critical step in battery production. It involves the preparation of electrode slurry, which contains active material, conductive additives, binder, and solvent. This slurry is then applied onto a foil (current collector) through a coating process. The quality of the electrode, its porosity, and the uniformity of the coating directly influence the battery cell’s performance, capacity, and lifespan.
The production of lithium-ion batteries requires precision in the calendaring process, which determines the electrode’s thickness and density. The choice of electrode materials, e.g., graphite for the anode and metal oxide for the cathode, and their respective mixing processes, play a significant role in the battery’s electrochemical performance. Any discrepancies in the electrode manufacturing can lead to reduced cell performance and even safety concerns.
What are the primary steps involved in the lithium-ion battery production line?
The lithium-ion battery production line involves several stages, starting from the preparation of electrode materials to the final cell assembly. Key processes include the mixing process to prepare electrode slurry, the coating process onto metal foils, the drying process, cell assembly, electrolyte filling, and battery testing. Each step is meticulously monitored to ensure the highest quality and performance of the final product.
The production of lithium-ion batteries is a continuous process, with line speed and precision being of utmost importance. From the slot die coater used in electrode manufacturing to the ultrasonic welding in cell assembly, every equipment and technique is chosen to optimize production efficiency and battery performance. Battery formation and testing equipment ensure that each cell meets the desired specifications before the final assembly into battery packs.
How do cell design and cell assembly vary in different types of lithium-ion batteries?
Lithium-ion batteries come in various designs, including cylindrical, prismatic, and pouch cells. Each design has its unique cell case, electrode configuration, and assembly process. For instance, cylindrical cells are stored in a metal casing, while pouch cells use a flexible, sealed pouch. The choice of design impacts the battery’s size, energy density, and thermal management.
Depending on the cell design, the manufacturing plant may employ different techniques for cell assembly, such as tab welding for connecting two electrodes in cylindrical cells. The cell finishing process, including sealing and degassing, also varies. For instance, pouch cells require a precise sealing process to ensure no electrolyte leakage. The choice between positive and negative electrodes, cathode and separator materials, and other components can also differ based on the specific cell design and intended application.
Why is battery testing crucial in the Li-ion battery production process?
Battery testing is an integral part of the Li-ion battery production process. It ensures that each battery cell meets the desired performance, safety, and quality standards. Tests assess various parameters, including capacity, voltage, internal resistance, and potential defects. This step is vital to identify and discard any subpar cells before they reach consumers.
In the realm of Li-ion battery production, testing equipment is used to simulate real-world conditions, assessing the battery’s state of charge, self-discharge rate, and overall electrochemical performance. Battery formation, which involves initial charging and discharging, is also a form of testing to ensure the cell’s stability. Continuous advancements in testing methodologies aim at reducing the cost of production while enhancing the safety and reliability of lithium-ion batteries.
No related posts.